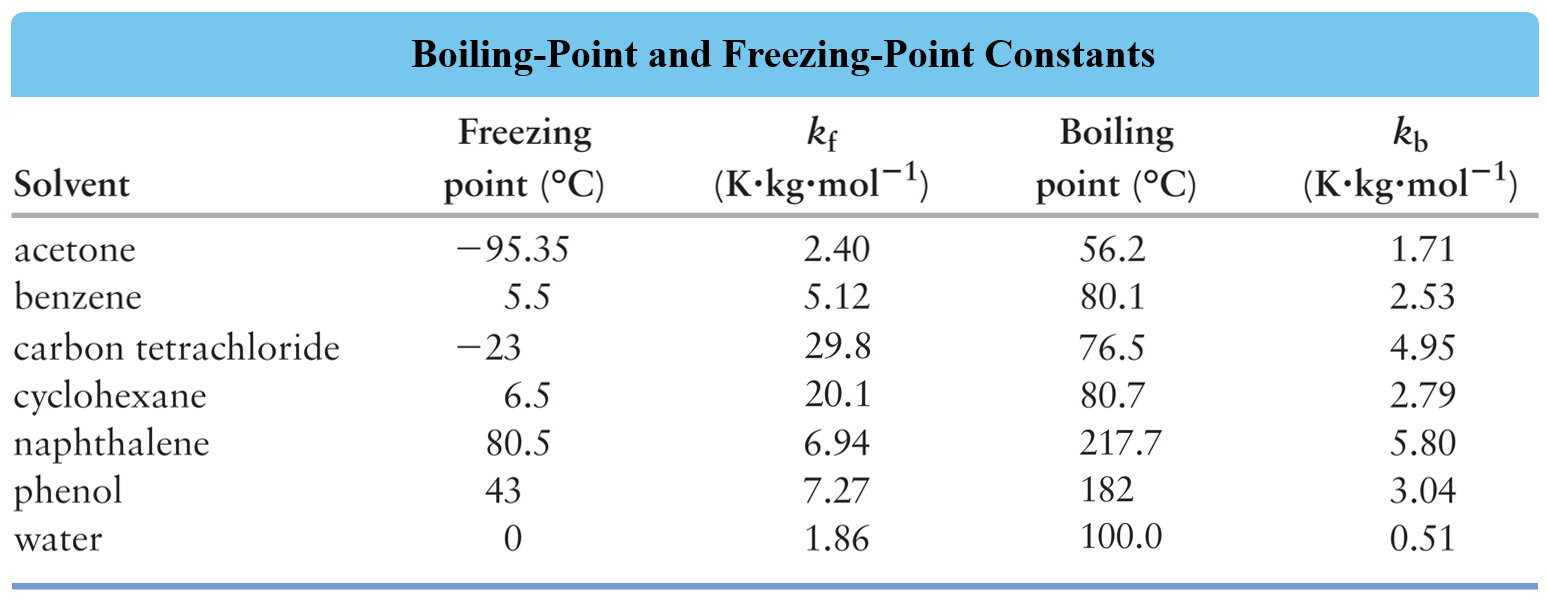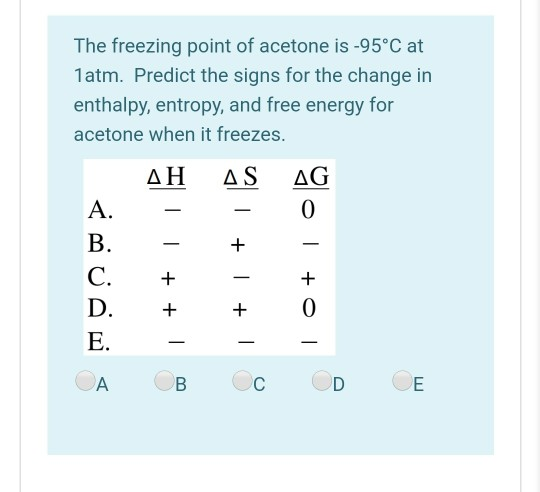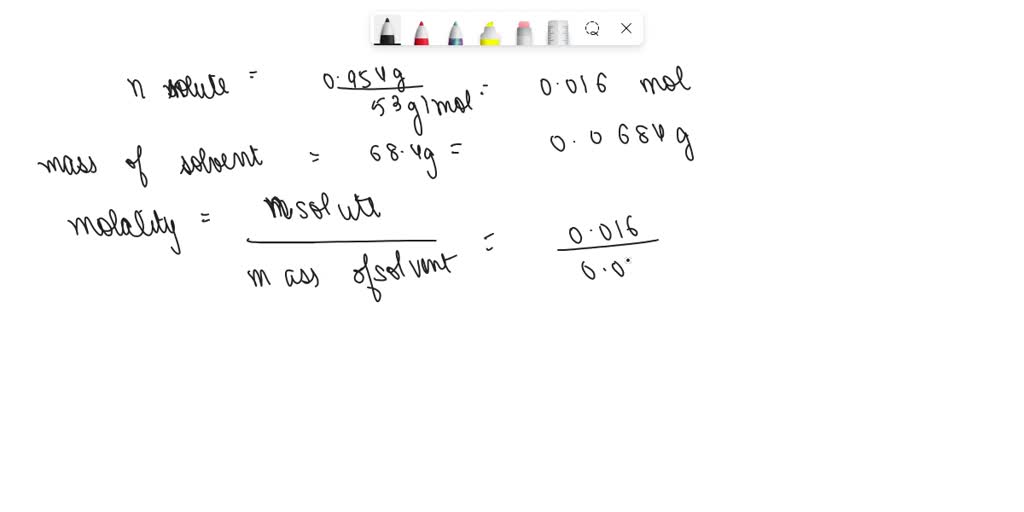
SOLVED: B) For D2O, the normal freezing point is 3.82°C and ΔHfus = 6305 J/mol. Find kf for D2O and find the freezing point of a solution of 0.954 g of acetone
Development of purity certified reference materials for methanol, ethanol, acetonitrile, acetone, ethyl acetate and n-hexane by freezing point depression - Analytical Methods (RSC Publishing)
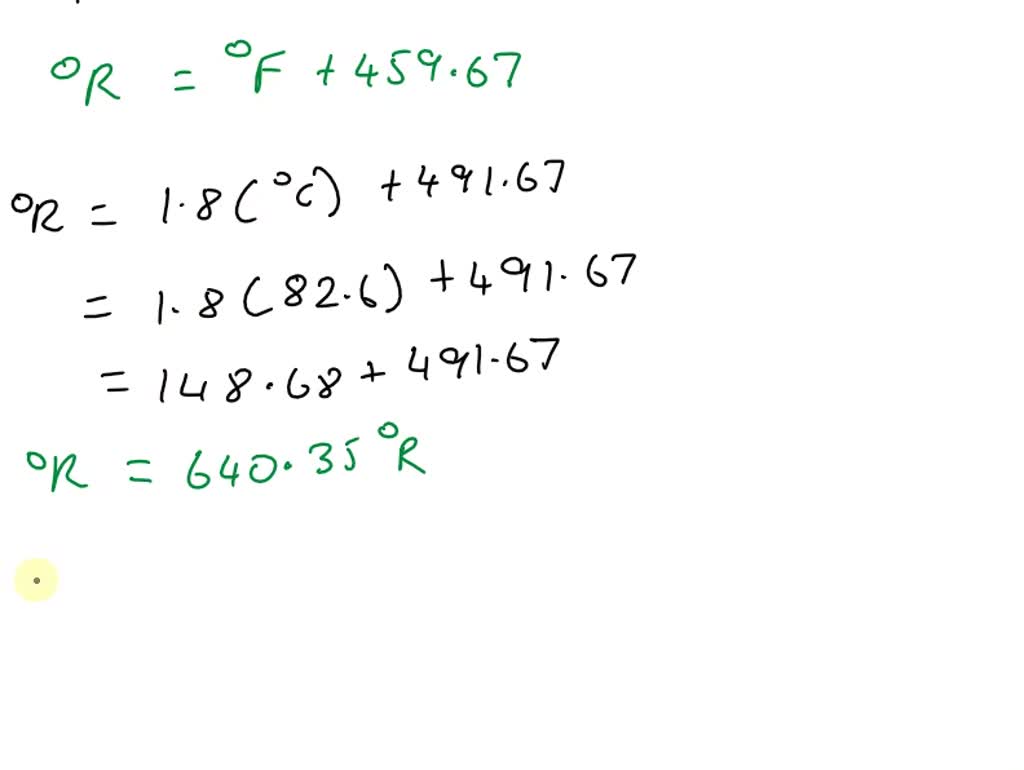
SOLVED: The freezing point and boiling point of acetone are 178.5 K and 329.20 K, respectively, or -138.5°F and 132.89°F, or 321.21°Ra and 592.56°Ra on the Rankine scale temperature scale. Derive an

thermodynamics - What mixing ratio of ethanol and acetone has the lowest freezing point? - Chemistry Stack Exchange
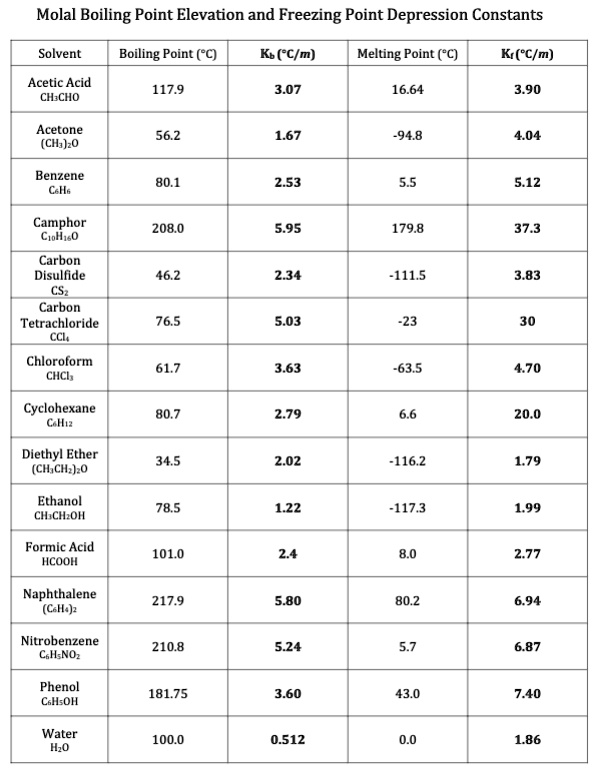
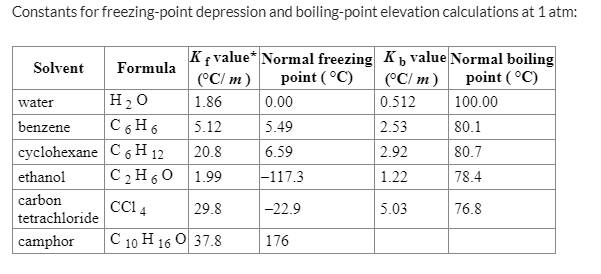
SOLVED: Molal Boiling Point Elevation and Freezing Point Depression Constants Solvent Boiling Point (°C) Kb (°C/m) Melting Point (°C) Kf (°C/m) Acetic Acid CH3COOH 117.9 3.07 16.64 3.90 Acetone (CH3)2CO 56.2 1.67 -

thermodynamics - What mixing ratio of ethanol and acetone has the lowest freezing point? - Chemistry Stack Exchange

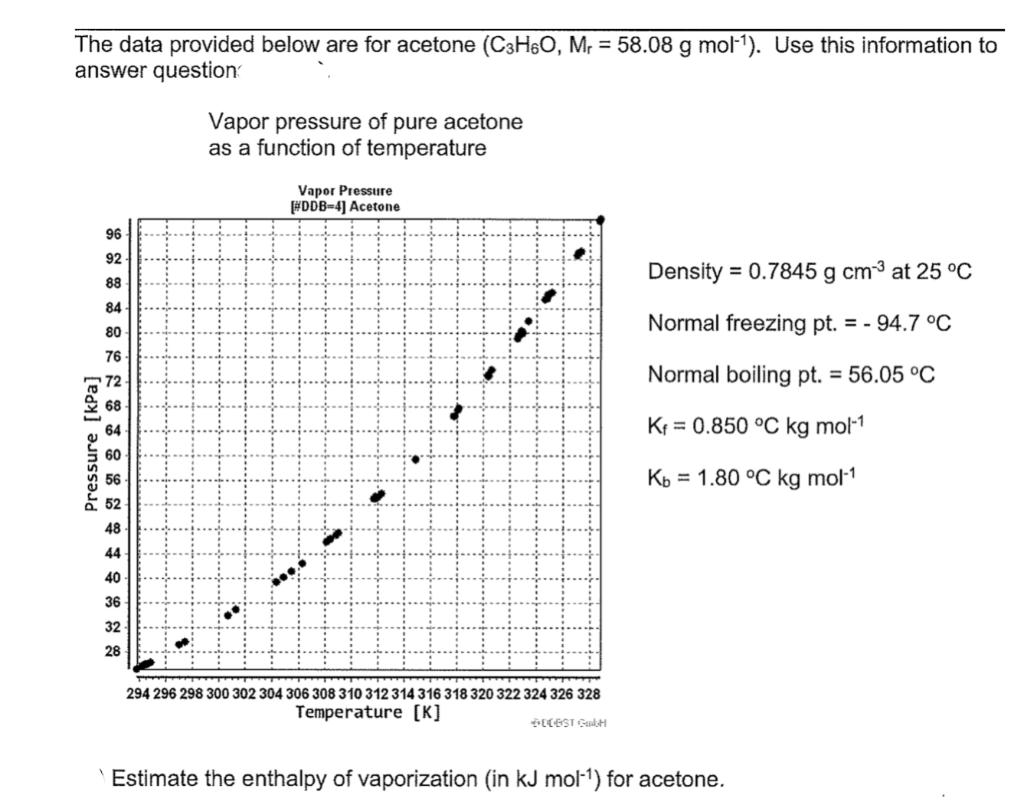
SOLVED: 016 Heating curve multi-part calculation 9 Points Use the table of thermodynamic properties for acetone to answer the following questions. Acetone's chemical formula is C3H6O and it has a molar mass

1-Butanol Separation from Aqueous Acetone-Butanol-Ethanol (ABE) Solutions by Freeze Concentration | Crystal Growth & Design
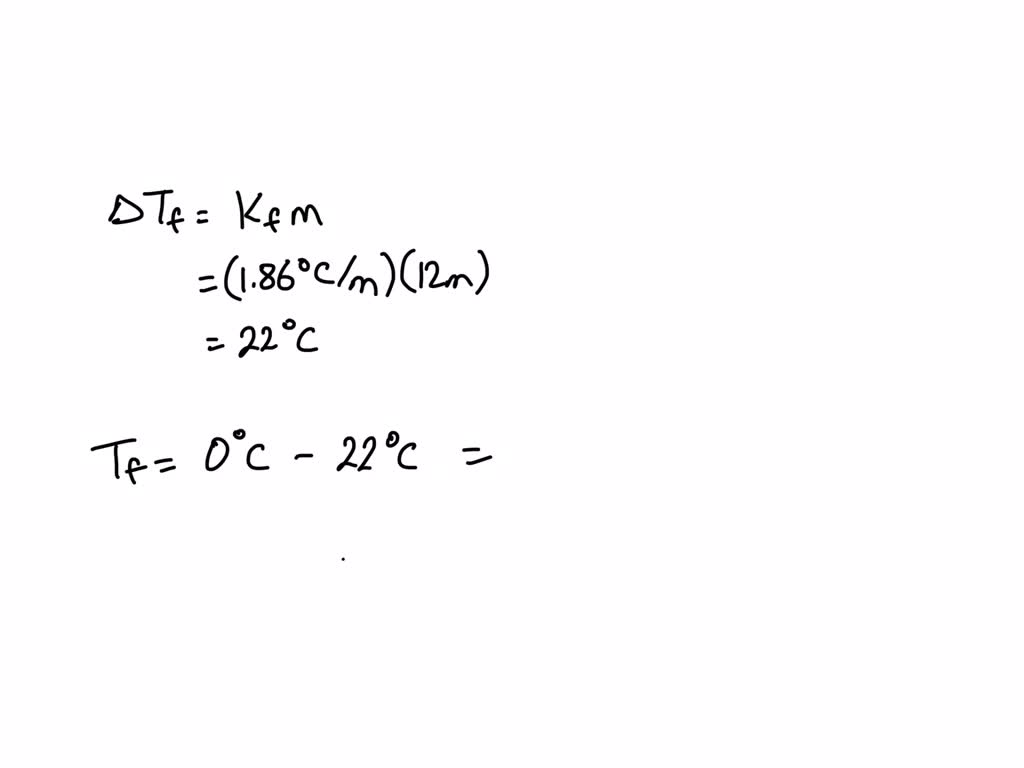
SOLVED: Calculate the freezing point of a 12.00 m aqueous solution of acetone. Freezing point constants can be found in the list of colligative constants. T f = ° C

10: Determination of the Molar Mass by Freezing Point Depression (Experiment) - Chemistry LibreTexts
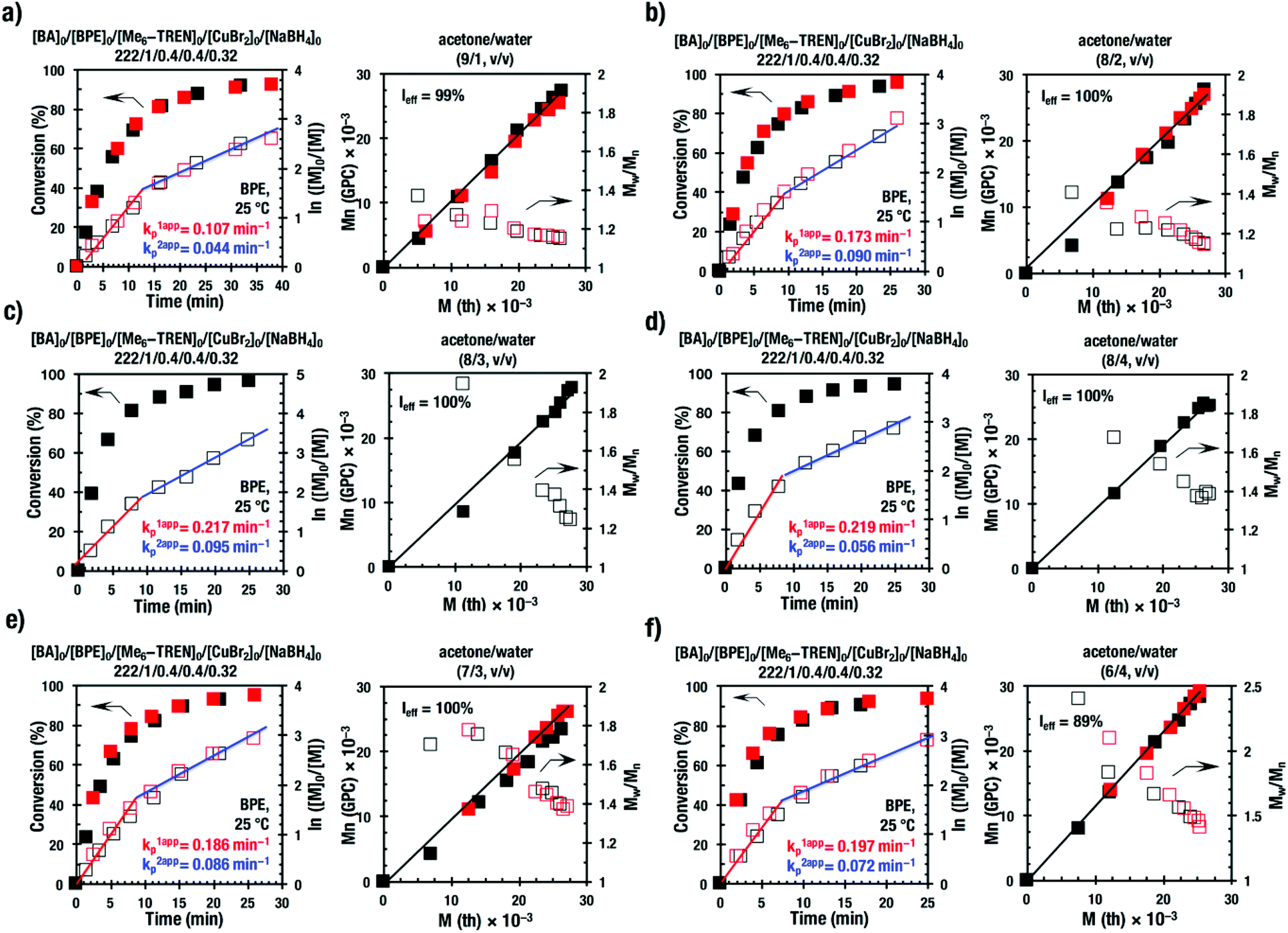
Acetone–water biphasic mixtures as solvents for ultrafast SET-LRP of hydrophobic acrylates - Polymer Chemistry (RSC Publishing) DOI:10.1039/C7PY00557A