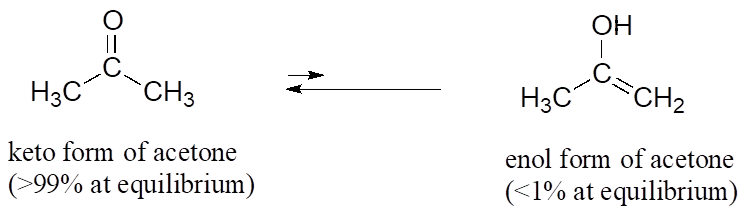
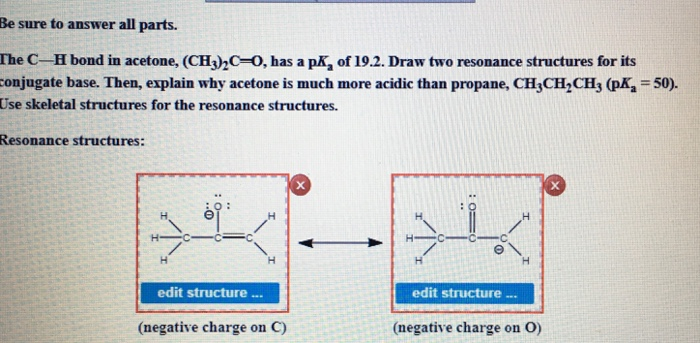
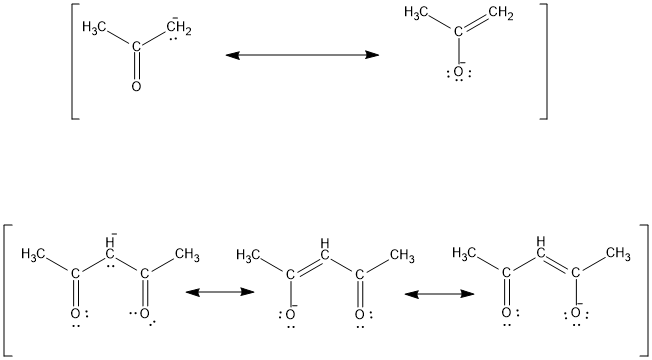
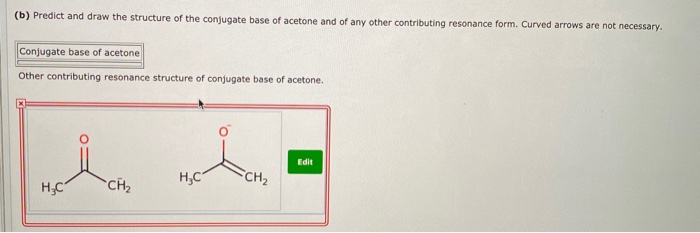
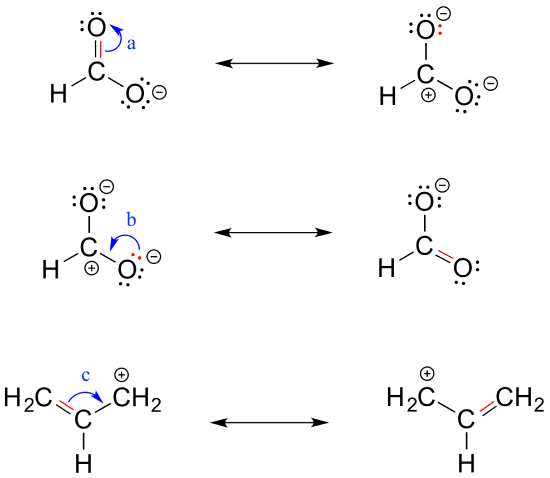
the c―h bond in acetone, (ch3)2c═o, has a pka of 19.2. draw two resonance structures for its conjugate - brainly.com

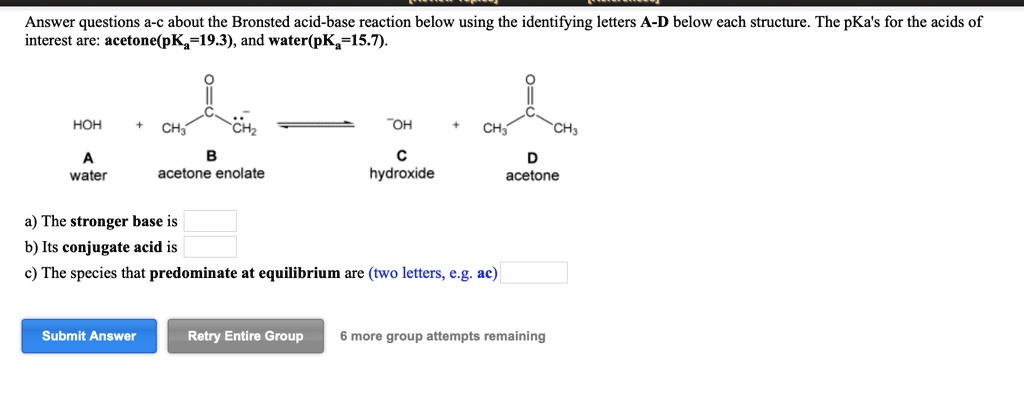
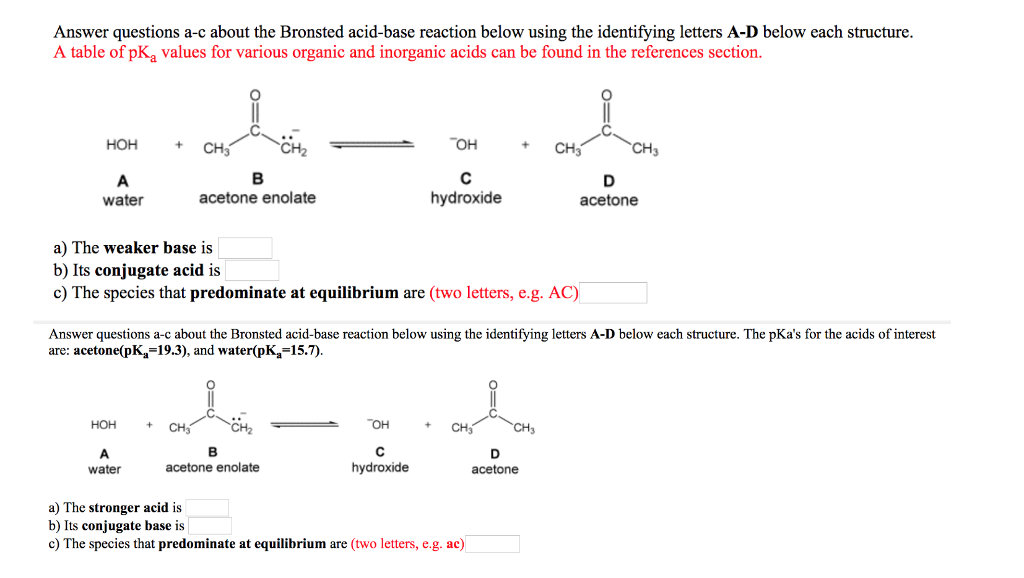
OneClass: Answer questions a-c about the Bronsted acid-base reaction below using the identifying lett...

SOLVED: " Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. The pKa's for the acids of interest are: acetone (pKa 19.3), and hydrogen

Write a structural formula of the conjugate acid formed by the reaction of CH3CH2OH with HCl. | Homework.Study.com

organic chemistry - Why is H2O a weaker acid than acetylacetone? Shouldn't a hydrogen connected to an oxygen be more acidic than a hydrogen connected to a carbon atom? - Chemistry Stack

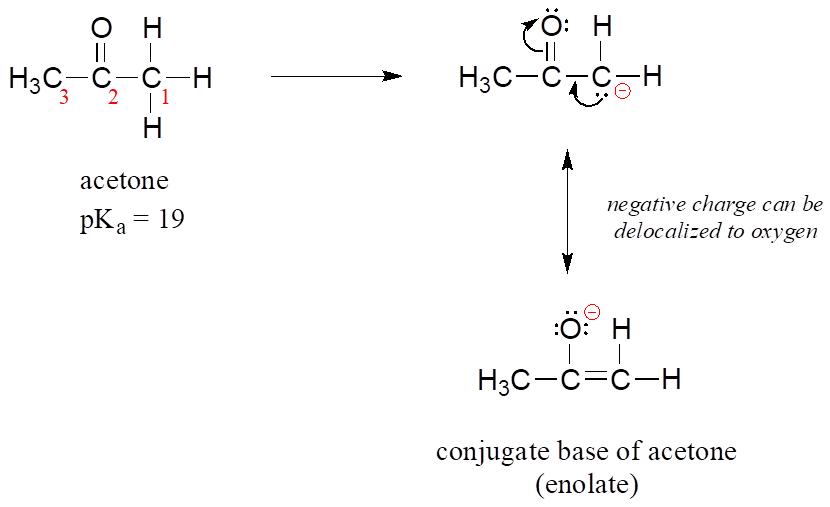
2,4-Pentanedione is a considerably stronger acid than is acetone. Write a structural formula for the conjugate base of each acid and account for the greater stability of the conjugate base from 2,4-pentanedione.