Applied Sciences | Free Full-Text | Effect of Ionic Liquids on the Isobaric Vapor-Liquid Equilibrium Behavior of Acetone-Chloroform

Control of the Maximum-Boiling Acetone/Chloroform Azeotropic Distillation System | Industrial & Engineering Chemistry Research

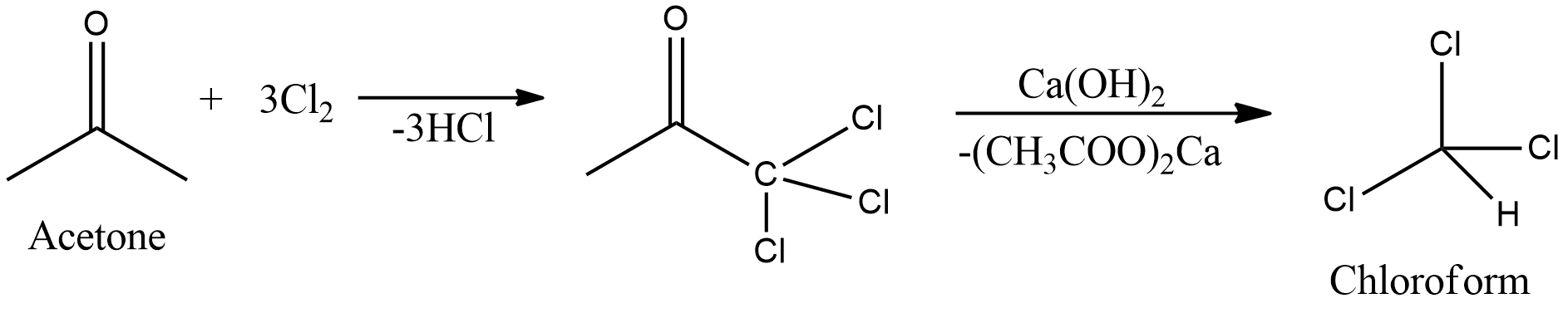
Lu Le Laboratory: Synthesis of Chloroform from Acetone and Bleach - Haloform Reaction - Lu Le Laboratory
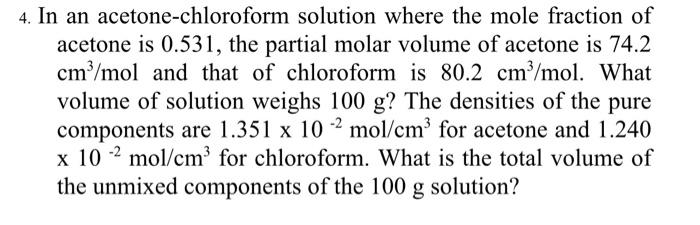
Which of the following reactions with chloroform will give chloretone ? A. $HN{O_3}$B. ${\\left( {C{H_3}} \\right)_2}C = O$C. Chloral D. ${\\left( {C{H_3}} \\right)_2}CHCHO$

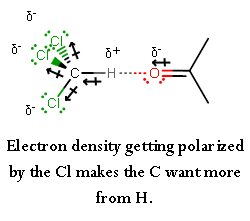
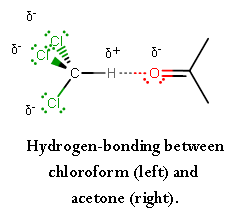
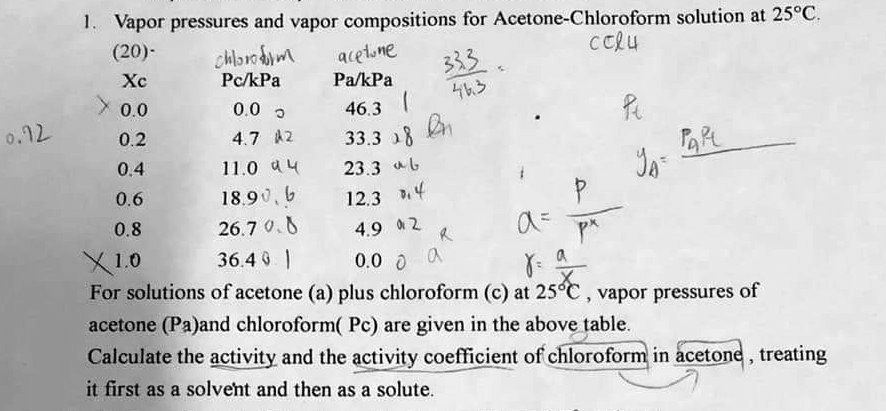
Chemistry Practical Class 12 Viva Questions on Determination of enthalpy change during interaction (Hydrogen bond formation) between acetone and chloroform.
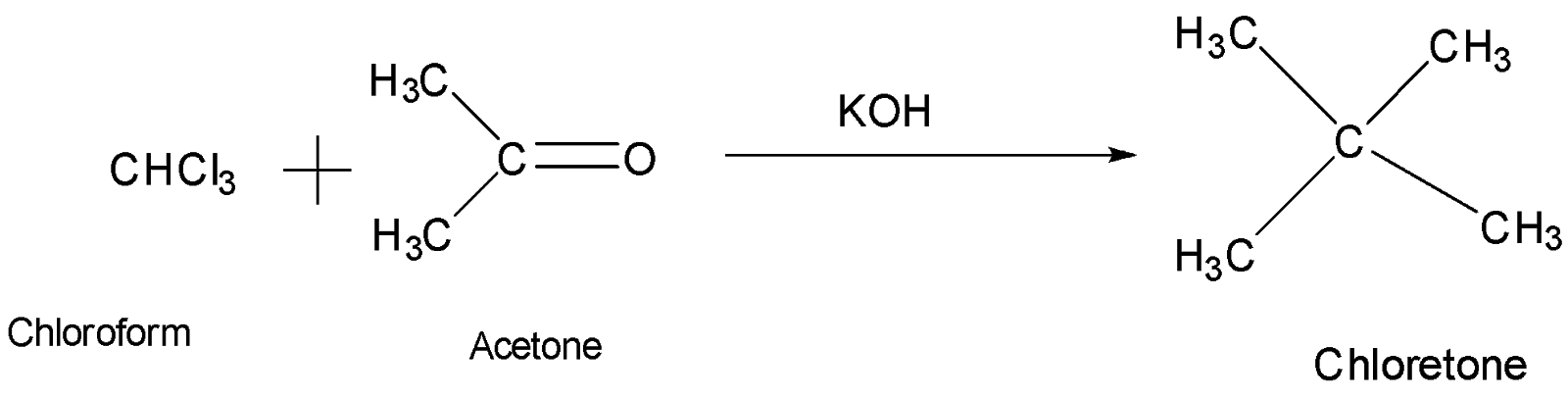
SOLVED: Vapor pressures and vapor compositions for Acetone-Chloroform solution at 25°C (20) - acetone cclu chloroform 333 Xc Pc/kPa Pa/kPa 463 X0.0 0.0 46.3 0.12 0.2 4.742 33.38 PaRt 0.4 11.04 23.3
Applied Sciences | Free Full-Text | Effect of Ionic Liquids on the Isobaric Vapor-Liquid Equilibrium Behavior of Acetone-Chloroform
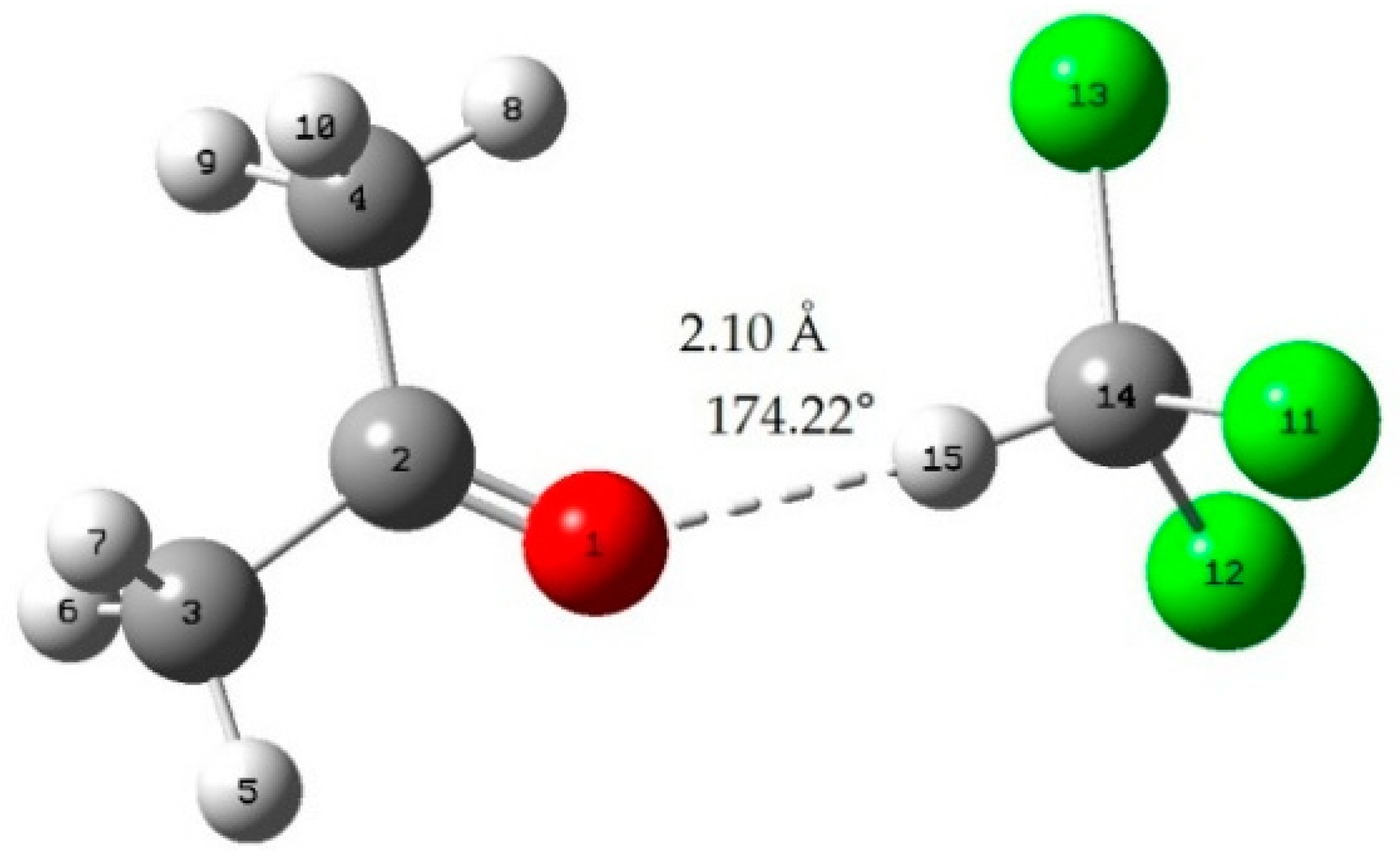
Molecular structure of (a) ABS, (b) acetone, (3) PC, and (d) chloroform. | Download Scientific Diagram

The liquid pair of acetone-chloroform shows a positive deviation form Raoult's law. | 12 | SOLUT... - YouTube

Comparison of extractive distillation and pressure-swing distillation for acetone/chloroform separation - ScienceDirect

Figure 2 from Separation of Acetone-chloroform maximum boiling azeotrope using Dimethyl sulfoxide | Semantic Scholar
Why does solution of chloroform & acetone show negative deviation from Raoult's law? Illustrate the deviation with the of diagrams.